More eyedrops recalled as FDA warns of ‘life-threatening infection’
Dr. Berne’s Whole Health is voluntarily recalling a selection of its eyedrops for fear they may be contaminated by bacteria and fungi.
The recall, which was shared last week by the Food and Drug Administration, includes the company’s MSM Drops 5% and 15% solutions; Dr. Berne’s Organic Castor Oil Eye Drops; and Dr. Berne’s MSM Mist 15% Solution.
An FDA analysis found one batch (lot 6786) of Dr. Berne’s MSM Drops 5% Solution showed bacterial and fungal contamination.
Out of an abundance of caution, Dr. Berne’s is recalling all of the other lots of the 5% and 15% strengths of MSM Solution and the other products.
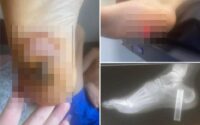
The FDA noted that using contaminated eyedrops could lead to “minor to serious” infections that could potentially affect vision and even “progress to a life-threatening infection.”



The FDA said Saturday that Dr. Berne’s has received two reports of adverse events related to the recall.
Consumers, distributors and retailers that have any of the recalled products should stop using them and return the products to Sun Star Organics, 988 N. Main St., Orange, California 92867.
The Post reached out to Dr. Berne’s for comment.
This is not the first time eyedrops have been recalled this year.
In January, the Centers for Disease Control and Prevention urged doctors and patients to stop using EzriCare Artificial Tears, as they had been linked to at least 55 infections in 12 states.

At the time, the product was blamed for cases of permanent vision loss, hospitalizations and one death.
“CDC recommends that clinicians and patients immediately discontinue the use of EzriCare Artificial Tears until the epidemiological investigation and laboratory analyses are complete,” read the January CDC statement.
By March, the CDC and FDA had identified 68 patients in 16 states who had been infected with a rare strain of bacteria after using the product.
Four people even had to have an eyeball surgically removed, while eight infections caused permanent vision loss.

Florida grandmother Clara Oliva claims she was declared legally blind after her right eye was surgically removed following use of EzriCare Artificial Tears.
“My client is horribly injured and now legally blind. I am currently investigating others similarly injured by this recalled product,” Oliva’s attorney, Natasha Cortes, wrote in an email to Law&Crime in March.
“These companies must be held accountable for the devastating consequences their product has caused Ms. Oliva and other consumers.”